How Many Protons In Na
iv
Be
Beryllium
Alkaline earth metal
12
Mg
Magnesium
Alkaline earth metal
xiii
Al
Aluminium
Post-transition metals
xx
Ca
Calcium
Alkaline earth metal
21
Sc
Scandium
Transition metals
22
Ti
Titanium
Transition metals
23
V
Vanadium
Transition metals
24
Cr
Chromium
Transition metals
25
Mn
Manganese
Transition metals
31
Ga
Gallium
Mail service-transition metals
38
Sr
Strontium
Alkaline world metals
xl
Zr
Zirconium
Transition metals
42
Mo
Molybdenum
Transition metals
43
Tc
Technetium
Transition metals
44
Ru
Ruthenium
Transition metals
46
Pd
Palladium
Transition metals
49
In
Indium
Post-transition metals
50
Sn
Tin
Post-transition metals
73
Ta
Tantalum
Transition metals
74
W
Tungsten
Transition metals
78
Pt
Platinum
Transition metals
81
Tl
Thallium
Post-transition metals
82
Atomic number 82
Pb
Mail-transition metals
83
Bi
Bismuth
Mail-transition metals
84
Po
Polonium
Mail service-transition metals
88
Ra
Radium
Alkali metal earth metal
104
Rf
Rutherfordium
Transition metal
106
Sg
Seaborgium
Transition element
What is Sodium
Sodium is a element with diminutive number11 which means there are 11 protons and 11 electrons in the atomic construction. Thechemical symbol for Sodium isNa.
Sodium is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, beingness in group 1 of the periodic tabular array, because it has a single electron in its outer shell that information technology readily donates, creating a positively charged atom—the Na+ cation.
Sodium – Properties
| Chemical element | Sodium |
|---|---|
| Atomic Number | xi |
| Symbol | Na |
| Chemical element Category | Alkaline |
| Phase at STP | Solid |
| Diminutive Mass [amu] | 22.9897 |
| Density at STP [grand/cm3] | 0.968 |
| Electron Configuration | [Ne] 3s1 |
| Possible Oxidation States | -one; +1 |
| Electron Affinity [kJ/mol] | 52.8 |
| Electronegativity [Pauling scale] | 0.93 |
| 1st Ionization Free energy [eV] | 5.1391 |
| Twelvemonth of Discovery | 1807 |
| Discoverer | Davy, Sir Humphry |
| Thermal backdrop | |
| Melting Indicate [Celsius calibration] | 97.viii |
| Boiling Point [Celsius calibration] | 883 |
| Thermal Expansion µm/(1000·G) | 71 |
| Thermal Conductivity [West/m K] | 141 |
| Specific Heat [J/g Chiliad] | 1.23 |
| Estrus of Fusion [kJ/mol] | 2.598 |
| Oestrus of Vaporization [kJ/mol] | 96.96 |
Atomic Number of Sodium
Sodium is a chemical element with diminutive number11 which means there are 11 protons and 11 electrons in the atomic construction. Thechemical symbol for Sodium isNa.
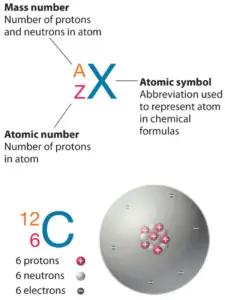 The cantlet consist of a modest but massivenucleus surrounded past a cloud of speedily movingelectrons. The nucleus is composed ofprotons and neutrons. Full number of protons in the nucleus is called theatomic number of the atom and is given thesymbol Z. The total electrical charge of the nucleus is therefore +Ze, where due east (elementary charge) equals toane,602 x ten-nineteen coulombs. In a neutral atom at that place are as many electrons as protons moving nigh nucleus. It is the electrons that are responsible for the chemical bavavior of atoms, and which identify the diverse chemical elements.
The cantlet consist of a modest but massivenucleus surrounded past a cloud of speedily movingelectrons. The nucleus is composed ofprotons and neutrons. Full number of protons in the nucleus is called theatomic number of the atom and is given thesymbol Z. The total electrical charge of the nucleus is therefore +Ze, where due east (elementary charge) equals toane,602 x ten-nineteen coulombs. In a neutral atom at that place are as many electrons as protons moving nigh nucleus. It is the electrons that are responsible for the chemical bavavior of atoms, and which identify the diverse chemical elements.
Encounter also: Atomic Number – Does information technology conserve in a nuclear reaction?
Diminutive Number and Chemical Properties
Every solid, liquid, gas, and plasma is equanimous of neutral or ionized atoms. Thechemical properties of the atom are adamant past the number of protons, in fact, past number and organisation of electrons. The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element's electron shells, specially the outermost valence beat, is the primary factor in determining its chemic bonding behavior. In the periodic table, the elements are listed in club of increasing diminutive number Z.
It is thePauli exclusion principle that requires the electrons in an atom to occupy different free energy levels instead of them all condensing in the ground country. The ordering of the electrons in the ground state of multielectron atoms, starts with the lowest free energy land (ground state) and moves progressively from there up the energy calibration until each of the atom'south electrons has been assigned a unique set of breakthrough numbers. This fact has key implications for the building up of the periodic table of elements.
Neutron Number and Mass Number of Sodium
Mass numbers of typical isotopes of Sodium are 23.
The total number of neutrons in the nucleus of an atom is called theneutron number of the cantlet and is given thesymbol North. Neutron number plus diminutive number equals atomic mass number:N+Z=A. The difference betwixt the neutron number and the atomic number is known equally theneutron excess: D = N – Z = A – 2Z.
Neutron number is rarely written explicitly in nuclide symbol note, but appears as a subscript to the correct of the element symbol. Nuclides that have the same neutron number but a different proton number are called isotones. The various species of atoms whose nuclei contain particular numbers of protons and neutrons are callednuclides. Each nuclide is denoted by chemic symbol of the element (this specifies Z) with tha atomic mass number as supescript. Therefore, nosotros cannot decide the neutron number of uranium, for example. We tin decide the neutron number of certain isotope. For example, the neutron number of uranium-238 is 238-92=146.
Neutron and Mass Numbers and Nuclear Properties
Properties of atomic nuclei (atomic mass, nuclear cross-sections) are determined past the number of protons and number of neutrons(neutron number). Information technology must be noted, specially nuclear cross-sections may vary by many orders from nuclide with the neutron number North to nuclide with the neutron number N+1. For example, actinides with odd neutron number are usually fissile (fissionable with dull neutrons) while actinides with even neutron number are usually not fissile (but are fissionable with fast neutrons). Heavy nuclei with an even number of protons and an even number of neutrons are (due to Pauli exclusion principle) very stable thanks to the occurrence of 'paired spin'. On the other hand, nuclei with an odd number of protons and neutrons are generally unstable.
Neutron and Diminutive Numbers and Nuclear Stability
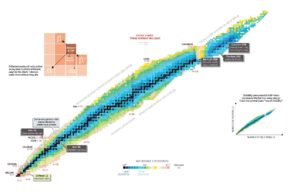
Nuclear stability is a concept that helps to identify the stability of an isotope. To place the stability of an isotope it is needed to find the ratio of neutrons to protons. To decide the stability of an isotope you tin can use the ratio neutron/proton (N/Z). Likewise to help understand this concept at that place is a chart of the nuclides, known as a Segre chart. This chart shows a plot of the known nuclides equally a function of their atomic and neutron numbers. It can be observed from the nautical chart that there aremore neutrons than protons in nuclides withZ greater than most xx (Calcium). Theseextra neutrons are necessary for stability of the heavier nuclei. The excess neutrons act somewhat similar nuclear gum. Only two stable nuclides have fewer neutrons than protons: hydrogen-i and helium-3.
Diminutive nuclei consist of protons and neutrons, which attract each other throughthe nuclear strength, while protons repel each other viathe electrical forcefulness due to their positive accuse. These two forces compete, leading to diverse stability of nuclei. In that location are only certain combinations of neutrons and protons, which formsstable nuclei.
Neutrons stabilize the nucleus, considering they attract each other and protons , which helps starting time the electrical repulsion betwixt protons. Equally a result, as the number of protons increases,an increasing ratio of neutrons to protons is needed to class a stable nucleus. If at that place are too many or too few neutrons for a given number of protons, the resulting nucleus is non stable and it undergoes radioactive decay.Unstable isotopesdecay through various radioactive decay pathways, almost unremarkably alpha decay, beta decay, gamma decay or electron capture. Many other rare types of decay, such as spontaneous fission or neutron emission are known.
Atomic Mass of Sodium
Atomic mass of Sodium is 22.9897 u.
The diminutive mass is the mass of an cantlet. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. The diminutive mass is carried by the diminutive nucleus, which occupies simply nigh 10-12of the total volume of the atom or less, only it contains all the positive charge and at least 99.95% of the total mass of the atom. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance.
The size and mass of atoms are so small that the use of normal measuring units, while possible, is often inconvenient. Units of measure out accept been defined for mass and energy on the atomic calibration to make measurements more convenient to limited. The unit of measure for mass is the atomic mass unit of measurement (amu). I atomic mass unit is equal to one.66 x x-24 grams. Ane unified atomic mass unit of measurement isapproximately the mass of 1 nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.
For12C the diminutive mass is exactly 12u, since the atomic mass unit is defined from it. For other isotopes, the isotopic mass usually differs and is usually within 0.1 u of the mass number. For instance, 63Cu (29 protons and 34 neutrons) has a mass number of 63 and an isotopic mass in itsnuclear ground state is 62.91367 u.
There are two reasons for the difference between mass number and isotopic mass, known as the mass defect:
- Theneutron is slightly heavier than theproton. This increases the mass of nuclei with more neutrons than protons relative to the atomic mass unit scale based on12C with equal numbers of protons and neutrons.
- The nuclear binding energy varies between nuclei. A nucleus with greater binding energy has a lower total energy, and therefore alower mass co-ordinate to Einstein's mass-energy equivalence relationE =mc 2. For 63Cu the diminutive mass is less than 63 so this must be the dominant cistron.
Note that, it was found therest mass of an atomic nucleusis measurably smaller thanthe sum of the residual masses of its elective protons, neutrons and electrons. Mass was no longer considered unchangeable in the closed system. The difference is a measure out of thenuclear binding energy which holds the nucleus together. According to the Einstein relationship (Eastward=mc2), this binding energy is proportional to thismass difference and information technology is known as themass defect.
Encounter also: Atomic Mass Number – Does it conserve in a nuclear reaction?
Atomic Radius of Sodium
The atomic radius of Sodium atom is 166pm (covalent radius).
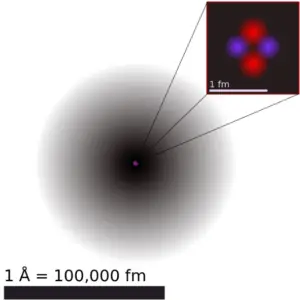
It must be noted, atoms lack a well-divers outer purlieus. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. However, this assumes the cantlet to showroom a spherical shape, which is merely obeyed for atoms in vacuum or free space. Therefore, there are various non-equivalent definitions of atomic radius.
- Van der Waals radius.In principle, Vana der Waals radius is one-half the minimum distance between the nuclei of ii atoms of the chemical element that are not jump to the same molecule.
- Ionic radius. An ionic radius is half the distance betwixt the nuclei of two ions in an ionic bond.
- Covalent radius. Covalent radius is the nominal radius of the atoms of an element when covalently bound to other atoms.
- Metallic radius. A metal radius is half the altitude between the nuclei of two adjacent atoms in a crystalline construction, when joined to other atoms by metallic bonds.
On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). Consequently, the smallest atom is helium with a radius of 32 pm, while one of the largest is caesium at 225 pm. The atomic radii decrease across the periodic table because as the atomic number increases, the number of protons increases across the period, but the extra electrons are only added to the same quantum shell. Therefore, the effective nuclear charge towards the outermost electrons increases, drawing the outermost electrons closer. As a event, the electron cloud contracts and the atomic radius decreases.
 The volume of an cantlet is aboutfifteen orders of magnitudelargerthan the volume of a nucleus. Foruranium atom, theVan der Waals radius is about186 pm = 1.86 ×x−10m. The Van der Waals radius, rw, of an cantlet is the radius of an imaginary hard sphere representing the altitude of closest arroyo for another atom. Assuming spherical shape, the uranium cantlet have book of about26.ix ×10−30 m3 . But this "huge" space is occupied primarily by electrons, because thenucleus occupies simply almost1721×10−45 k3 of space. These electrons together weigh only a fraction (allow say 0.05%) of entire atom.
The volume of an cantlet is aboutfifteen orders of magnitudelargerthan the volume of a nucleus. Foruranium atom, theVan der Waals radius is about186 pm = 1.86 ×x−10m. The Van der Waals radius, rw, of an cantlet is the radius of an imaginary hard sphere representing the altitude of closest arroyo for another atom. Assuming spherical shape, the uranium cantlet have book of about26.ix ×10−30 m3 . But this "huge" space is occupied primarily by electrons, because thenucleus occupies simply almost1721×10−45 k3 of space. These electrons together weigh only a fraction (allow say 0.05%) of entire atom.
Information technology may seem, that the space and in fact the matter isempty,but information technology is non. Due to thequantum nature of electrons, the electrons are not bespeak particles, they are smeared out over the whole atom. The classical description cannot be used to describe things on the diminutive scale. On the diminutive calibration, physicists take institute that quantum mechanics describes things very well on that calibration. Particle locations in quantum mechanics are non at an exact position, they are described by aprobability density function. Therefore the space in an cantlet (betwixt electrons and an atomic nucleus) is not empty, but it is filled past a probability density function of electrons (unremarkably known as "electron cloud").
 Density of Sodium
Density of Sodium
Density of Sodium is0.968g/cm3 .
Typical densities of various substances are at atmospheric pressure.
Density is divers equally themass per unit of measurement volume. Information technology is anintensive property, which is mathematically defined as mass divided past volume:
ρ = m/V
In words, the density (ρ) of a substance is the total mass (yard) of that substance divided by the total volume (5) occupied by that substance. The standard SI unit iskilograms per cubic meter (kg/g3 ). The Standard English language unit ispounds mass per cubic foot (lbm/ft3 ).
Density – Atomic Mass and Atomic Number Density
Since the density (ρ) of a substance is the total mass (m) of that substance divided past the total volume (Five) occupied by that substance, it is obvious, the density of a substance strongly depends on its diminutive mass and too on the diminutive number density (N; atoms/cm3),
- Atomic Weight. The atomic mass is carried past the diminutive nucleus, which occupies only about 10-12of the total volume of the cantlet or less, but it contains all the positive charge and at to the lowest degree 99.95% of the total mass of the atom. Therefore it is adamant by the mass number (number of protons and neutrons).
- Atomic Number Density. The atomic number density (North; atoms/cm3), which is associated with diminutive radii, is the number of atoms of a given type per unit volume (V; cmthree) of the fabric. The atomic number density (N; atoms/cm3) of a pure textile havingdiminutive or molecular weight (Chiliad; grams/mol) and thematerial density (⍴; gram/cm3) is easily computed from the following equation using Avogadro's number (NA = half dozen.022×x23 atoms or molecules per mole):
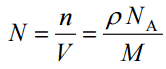
Sincenucleons (protons andneutrons) make upward nearly of the mass of ordinary atoms, the density of normal matter tends to be limited by how closely we can pack these nucleons and depends on the internal atomic structure of a substance. Thedensest textile found on earth is themetal osmium, simply its density pales by comparing to the densities of exotic astronomical objects such as white dwarf stars andneutron stars.
If we include human made elements, the densest so far is Hassium.Hassium is a element with symbolHs and diminutive number 108. Information technology is a constructed element (first synthesised at Hasse in Deutschland) and radioactive. The most stable known isotope, 269Hs, has a one-half-life of approximately 9.7 seconds. It has an estimated density of 40.7 x 103 kg/m3 . The density of Hassium results from itshigh atomic weight and from the significant decrease inionic radii of the elements in the lanthanide series, known equallylanthanide and actinide contraction.
Density – Pressure level and Temperature
The density of a material varies with temperature and pressure. This variation is typically small for solids and liquids but much greater for gases. Most materials expand when their temperatures increase. Rising temperatures brand the liquid aggrandize in a liquid-in-tube thermometer and bend bimetallic strips. As a result of this expansion, the density of most materials decreases. This result is caused by a decrease in the diminutive number density. This dependence is unremarkably expressed by the coefficient of linear or volume expansion.
Increasing the pressure level on an cloth (peculiarly for liquids or gases) decreases the book of the object and thus increases its density via the diminutive number density. Compressibility (besides known as the coefficient of compressibility is a measure out of the relative book change of a fluid or solid as a response to a pressure (or mean stress) change.
See also: What is Density
Come across as well: Densest Materials of the World
Electron Configuration and Oxidation States of Sodium
Electron configuration of Sodiumis [Ne] 3s1.
Possible oxidation states are -1; +ane.
Electron Configuration
The periodic table is a tabular brandish of the chemic elements organized on the basis of their atomic numbers, electron configurations, and chemical properties. The electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. Knowledge of the electron configuration of different atoms is useful in understanding the construction of the periodic table of elements.
Every solid, liquid, gas, and plasma is equanimous of neutral or ionized atoms. Thechemical properties of the atom are determined by the number of protons, in fact, by number and organization of electrons. The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element'southward electron shells, particularly the outermost valence shell, is the principal cistron in determining its chemical bonding behavior. In the periodic table, the elements are listed in lodge of increasing atomic number Z.
It is thePauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. The ordering of the electrons in the ground country of multielectron atoms, starts with the everyman energy state (ground country) and moves progressively from there up the free energy scale until each of the atom'southward electrons has been assigned a unique set of quantum numbers. This fact has cardinal implications for the edifice upwardly of the periodic table of elements.
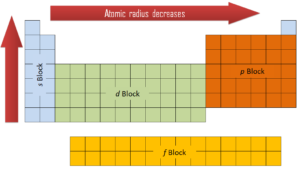
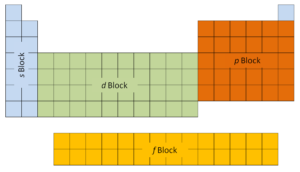 The outset 2 columns on the left side of the periodic table are where thesouthward subshells are being occupied. Because of this, the first 2 rows of the periodic table are labeled thes cake. Similarly, thep blockare the right-most half-dozen columns of the periodic tabular array, thed blockis the middle x columns of the periodic table, while thef cakeis the 14-column section that is unremarkably depicted as detached from the primary body of the periodic table. Information technology could be office of the main body, but then the periodic table would be rather long and cumbersome.
The outset 2 columns on the left side of the periodic table are where thesouthward subshells are being occupied. Because of this, the first 2 rows of the periodic table are labeled thes cake. Similarly, thep blockare the right-most half-dozen columns of the periodic tabular array, thed blockis the middle x columns of the periodic table, while thef cakeis the 14-column section that is unremarkably depicted as detached from the primary body of the periodic table. Information technology could be office of the main body, but then the periodic table would be rather long and cumbersome.
For atoms with many electrons, this notation can go lengthy and then an abbreviated annotation is used. The electron configuration can exist visualized as the cadre electrons, equivalent to the noble gas of the preceding menstruation, and the valence electrons (e.grand. [Xe] 6s2 for barium).
Oxidation States
Oxidation states are typically represented by integers which may be positive, zero, or negative. Most elements have more than than 1 possible oxidation state. For instance, carbon has nine possible integer oxidation states from −4 to +4.
The current IUPAC Gilded Book definition of oxidation state is:
"Oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds…"
and the term oxidation number is well-nigh synonymous. An element that is not combined with any other different elements has an oxidation state of 0. Oxidation state 0 occurs for all elements – it is simply the element in its elemental form. An cantlet of an chemical element in a compound volition have a positive oxidation country if information technology has had electrons removed. Similarly, adding electrons results in a negative oxidation country. We have besides distinguish betwixt the possible and common oxidation states of every element. For instance, silicon has nine possible integer oxidation states from −four to +4, but simply -iv, 0 and +4 are common oxidation states.
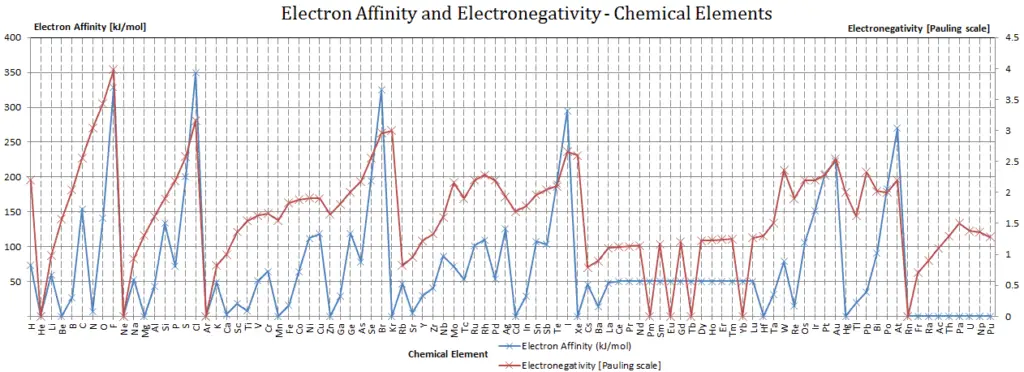
Electron Affinity and Electronegativity of Sodium
Electron affinity of Sodium is 52.8 kJ/mol.
Electronegativity of Sodium is0.93.
Electron Affinity
In chemistry and atomic physics, theelectron analogousness of an cantlet or molecule is defined every bit:
the change in energy (in kJ/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the cantlet to form a negative ion.
10 + e– → X– + energy Affinity = – ∆H
In other words, it can exist expressed as the neutral cantlet'slikelihood of gaining an electron. Note that, ionization energies measure the tendency of a neutral atom to resist the loss of electrons. Electron affinities are more difficult to measure than ionization energies.
An cantlet of Sodium in the gas phase, for instance, gives off energy when it gains an electron to grade an ion of Sodium.
Na + e– → Na– – ∆H = Affinity = 52.8 kJ/mol
To utilize electron affinities properly, it is essential to keep rails of sign. When an electron is added to a neutral atom, free energy is released. This analogousness is known as the first electron affinity and these energies are negative. By convention, the negative sign shows a release of energy. However, more energy is required to add an electron to a negative ion which overwhelms any the release of free energy from the electron attachment process. This affinity is known every bit the second electron analogousness and these energies are positive.
 Electron affinity tin can be either positive or negative value. The greater the negative value, the more than stable the anion is. Although analogousness varies profoundly beyond the periodic table, some patterns emerge. By and large, the elements on the right side of the periodic table will accept large negative electron analogousness. The electron affinities will become less negative as you lot go from the top to the bottom of the periodic table. Still, nitrogen, oxygen, and fluorine do not follow this trend. Moreover, nonmetals accept more positive affinity than metals. Atoms whose anions are more than stable than neutral atoms have a greater affinity. Chlorine virtually strongly attracts actress electrons, while neon most weakly attracts an extra electron.
Electron affinity tin can be either positive or negative value. The greater the negative value, the more than stable the anion is. Although analogousness varies profoundly beyond the periodic table, some patterns emerge. By and large, the elements on the right side of the periodic table will accept large negative electron analogousness. The electron affinities will become less negative as you lot go from the top to the bottom of the periodic table. Still, nitrogen, oxygen, and fluorine do not follow this trend. Moreover, nonmetals accept more positive affinity than metals. Atoms whose anions are more than stable than neutral atoms have a greater affinity. Chlorine virtually strongly attracts actress electrons, while neon most weakly attracts an extra electron.
Affinities of Non metals vs. Affinities of Metals
- Metals: Metals like to lose valence electrons to form cations to have a fully stable shell. The electron affinity of metals is lower than that of nonmetals. Mercury nigh weakly attracts an extra electron.
- Nonmetals: Generally, nonmetals have more positive electron affinity than metals. Nonmetals like to proceeds electrons to course anions to have a fully stable electron shell. Chlorine most strongly attracts extra electrons. The electron affinities of the noble gases have not been conclusively measured, so they may or may not have slightly negative values.
Electronegativity
Electronegativity, symbol χ, is a chemical property that describes the trend of an cantlet to concenter electrons towards this atom. For this purposes, adimensionless quantity thePauling scale, symbol χ, is the most commonly used.
The electronegativity of Sodium is: χ = 0.93
 In general, an atom'due south electronegativity is affected by both its diminutive number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity number, the more an chemical element or compound attracts electrons towards it. The most electronegative atom, fluorine, is assigned a value of four.0, and values range down to cesium and francium which are the least electronegative at 0.7. Electronegativity is related with ionization energy and electron analogousness. Electrons with low ionization energies have depression electronegativities because their nuclei do not exert a strong attractive forcefulness on electrons. Elements with high ionization energies have high electronegativities due to the strong pull exerted past the positive nucleus on the negative electrons. Therefore the electronegativity is greatest at the summit-right of the periodic table and decreases toward the bottom-left.
In general, an atom'due south electronegativity is affected by both its diminutive number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity number, the more an chemical element or compound attracts electrons towards it. The most electronegative atom, fluorine, is assigned a value of four.0, and values range down to cesium and francium which are the least electronegative at 0.7. Electronegativity is related with ionization energy and electron analogousness. Electrons with low ionization energies have depression electronegativities because their nuclei do not exert a strong attractive forcefulness on electrons. Elements with high ionization energies have high electronegativities due to the strong pull exerted past the positive nucleus on the negative electrons. Therefore the electronegativity is greatest at the summit-right of the periodic table and decreases toward the bottom-left.
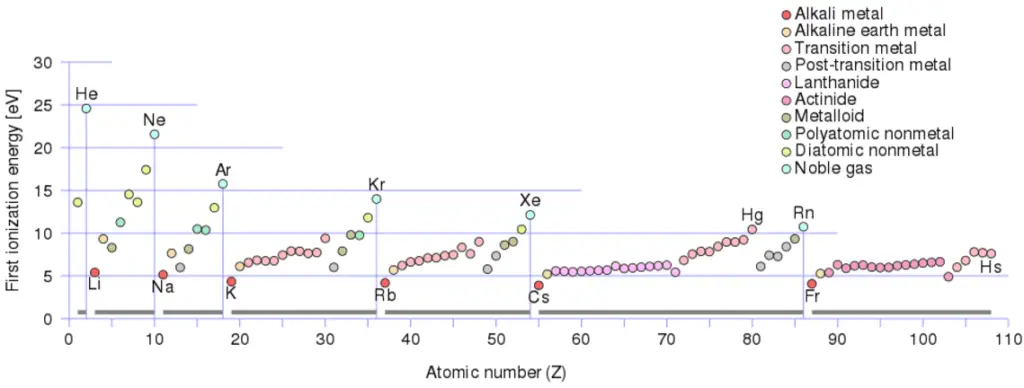
Beginning Ionization Free energy of Sodium
First Ionization Energy of Sodium is 5.1391 eV.
Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral atom.
X + energy → Ten+ + due east−
where Ten is any cantlet or molecule capable of beingness ionized, X+ is that cantlet or molecule with an electron removed (positive ion), and due east− is the removed electron.
A Sodium atom, for example, requires the following ionization energy to remove the outermost electron.
Na + IE → Na+ + due east− IE = 5.1391 eV
The ionization energy associated with removal of the first electron is most usually used. Thenth ionization energy refers to the corporeality of free energy required to remove an electron from the species with a charge of (n-1).
1st ionization energy
10 → X+ + e−
2nd ionization energy
X+ → X2+ + due east−
3rd ionization energy
Xtwo+ → X3+ + eastward−
Ionization Free energy for dissimilar Elements
In that location is an ionization energy for each successive electron removed. The electrons that circle the nucleus move in fairly well-defined orbits. Some of these electrons are more tightly bound in the atom than others. For instance, only 7.38 eV is required to remove the outermost electron from a atomic number 82 cantlet, while 88,000 eV is required to remove the innermost electron. Helps to understand reactivity of elements (especially metals, which lose electrons).
 In general, the ionization energy increases moving upwardly a group and moving left to right across a period. Ionization energy is is related with electronegativity and electron affinity. Electrons with low ionization energies have low electronegativities because their nuclei practise non exert a stiff attractive force on electrons. Elements with loftier ionization energies have high electronegativities due to the strong pull exerted past the positive nucleus on the negative electrons. Therefore the electronegativity is greatest at the top-right of the periodic table and decreases toward the bottom-left. Moreover:
In general, the ionization energy increases moving upwardly a group and moving left to right across a period. Ionization energy is is related with electronegativity and electron affinity. Electrons with low ionization energies have low electronegativities because their nuclei practise non exert a stiff attractive force on electrons. Elements with loftier ionization energies have high electronegativities due to the strong pull exerted past the positive nucleus on the negative electrons. Therefore the electronegativity is greatest at the top-right of the periodic table and decreases toward the bottom-left. Moreover:
- Ionization energy is lowest for the brine metals which take a single electron outside a closed shell.
- Ionization energy increases beyond a row on the periodic maximum for the noble gases which have airtight shells.
For example, sodium requires only 496 kJ/mol or v.14 eV/atom to ionize it. On the other hand neon, the noble gas, immediately preceding it in the periodic table, requires 2081 kJ/mol or 21.56 eV/atom.
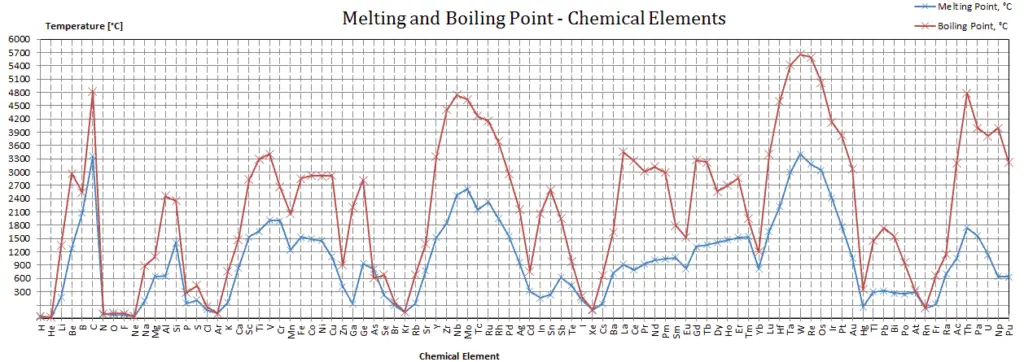
Sodium – Melting Point and Humid Point
Melting indicate of Sodium is 97.8°C.
Boiling point of Sodium is 883°C.
Note that, these points are associated with the standard atmospheric pressure level.
Boiling Point
In general, boiling is a phase alter of a substance from the liquid to the gas stage. The boiling indicate of a substance is the temperature at which this stage modify (humid or vaporization) occurs. The temperature at which vaporization (boiling) starts to occur for a given pressure is also known every bit the saturation temperature and at this weather condition a mixture of vapor and liquid tin be together. The liquid can be said to exist saturated with thermal free energy. Any addition of thermal energy results in a phase transition. At the boiling point the 2 phases of a substance, liquid and vapor, have identical free energies and therefore are equally likely to exist. Below the boiling indicate, the liquid is the more stable state of the two, whereas in a higher place the gaseous form is preferred. The pressure level at which vaporization (boiling) starts to occur for a given temperature is called the saturation force per unit area. When considered every bit the temperature of the reverse alter from vapor to liquid, it is referred to equally the condensation point.
As tin can exist seen, the boiling bespeak of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum has a lower humid point than when that liquid is at atmospheric pressure. A liquid at high pressure level has a higher boiling point than when that liquid is at atmospheric pressure. For example, water boils at 100°C (212°F) at body of water level, but at 93.4°C (200.one°F) at 1900 metres (6,233 ft) altitude. On the other hand, water boils at 350°C (662°F) at 16.5 MPa (typical pressure of PWRs).
In the periodic table of elements, the element with the lowest boiling signal is helium. Both the boiling points of rhenium and tungsten exceed 5000 Yard at standard pressure level. Since it is difficult to measure extreme temperatures precisely without bias, both have been cited in the literature equally having the higher boiling point.
Melting Indicate
In general, melting is a phase change of a substance from the solid to the liquid phase. The melting point of a substance is the temperature at which this phase change occurs. The melting signal also defines a condition in which the solid and liquid can be in equilibrium. Adding a estrus will convert the solid into a liquid with no temperature change. At the melting point the ii phases of a substance, liquid and vapor, have identical free energies and therefore are every bit likely to be. Below the melting bespeak, the solid is the more stable country of the two, whereas above the liquid form is preferred. The melting point of a substance depends on pressure and is normally specified at standard force per unit area. When considered as the temperature of the opposite alter from liquid to solid, it is referred to equally the freezing signal or crystallization point.
See also: Melting Betoken Depression
The first theory explaining mechanism of melting in the bulk was proposed by Lindemann, who used vibration of atoms in the crystal to explain the melting transition. Solids are similar to liquids in that both are condensed states, with particles that are far closer together than those of a gas. The atoms in a solid are tightly bound to each other, either in a regular geometric lattice (crystalline solids, which include metals and ordinary ice) or irregularly (an amorphous solid such as mutual window glass), and are typically low in energy. The motion of private atoms, ions, or molecules in a solid is restricted to vibrational motion well-nigh a fixed point. As a solid is heated, its particles vibrate more rapidly as the solid absorbs kinetic energy. At some point the aamplitude of vibration becomes and then big that the atoms kickoff to invade the infinite of their nearest neighbors and disturb them and the melting process initiates. The melting point is the temperature at which the confusing vibrations of the particles of the solid overcome the attractive forces operating inside the solid.
Every bit with boiling points, the melting point of a solid is dependent on the strength of those attractive forces. For example, sodium chloride (NaCl) is an ionic compound that consists of a multitude of strong ionic bonds. Sodium chloride melts at 801°C. On the other hand, ice (solid HtwoO) is a molecular compound whose molecules are held together by hydrogen bonds, which is effectively a strong example of an interaction between two permanent dipoles. Though hydrogen bonds are the strongest of the intermolecular forces, the force of hydrogen bonds is much less than that of ionic bonds. The melting betoken of ice is 0 °C.
Covalent bonds often effect in the germination of small collections of improve-connected atoms called molecules, which in solids and liquids are bound to other molecules by forces that are ofttimes much weaker than the covalent bonds that hold the molecules internally together. Such weak intermolecular bonds requite organic molecular substances, such as waxes and oils, their soft bulk character, and their depression melting points (in liquids, molecules must cease most structured or oriented contact with each other).
Sodium – Thermal Electrical conductivity
Thermal electrical conductivity of Sodium is 141 W/(thou·Grand).
The heat transfer characteristics of a solid material are measured by a belongings called the thermal electrical conductivity, k (or λ), measured in W/thousand.K. It is a measure of a substance's ability to transfer heat through a material by conduction. Note that Fourier's police force applies for all affair, regardless of its state (solid, liquid, or gas), therefore, it is also defined for liquids and gases.
The thermal conductivity of most liquids and solids varies with temperature. For vapors, it as well depends upon pressure. In full general:
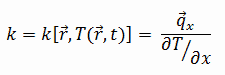
Most materials are very about homogeneous, therefore we can usually write thousand = k (T) . Similar definitions are associated with thermal conductivities in the y- and z-directions (ky, kz), but for an isotropic fabric the thermal conductivity is independent of the direction of transfer, kx = ky = kz = grand.
Thermal Conductivity of Metals
Transport of thermal energy in solids may be generally due to two furnishings:
- the migration of complimentary electrons
- lattice vibrational waves (phonons)
When electrons and phonons carry thermal energy leading to conduction heat transfer in a solid, the thermal electrical conductivity may be expressed as:
k = ke + yardph
 Metals are solids and equally such they possess crystalline structure where the ions (nuclei with their surrounding shells of core electrons) occupy translationally equivalent positions in the crystal lattice.Metals in general accepthigh electrical conductivity,high thermal conductivity, and high density. Appropriately, transport of thermal energy may be due to two furnishings:
Metals are solids and equally such they possess crystalline structure where the ions (nuclei with their surrounding shells of core electrons) occupy translationally equivalent positions in the crystal lattice.Metals in general accepthigh electrical conductivity,high thermal conductivity, and high density. Appropriately, transport of thermal energy may be due to two furnishings:
- the migration offree electrons
- lattice vibrational waves (phonons).
When electrons and phonons conduct thermal energy leading to conduction heat transfer in a solid, the thermal electrical conductivity may be expressed every bit:
k = yarddue east + chiliadph
The unique feature of metals as far every bit their construction is concerned is the presence of accuse carriers, specificallyelectrons. The electric and thermal conductivities of metalsoriginate from the fact that theirouter electrons are delocalized. Their contribution to the thermal conductivity is referred to as theelectronic thermal conductivity, k e . In fact, in pure metals such as gold, silver, copper, and aluminum, the rut current associated with the flow of electrons past far exceeds a small contribution due to the menstruation of phonons. In contrast, for alloys, the contribution of gph to one thousand is no longer negligible.
Thermal Conductivity of Nonmetals
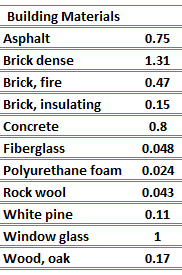 For nonmetallic solids,thou is adamant primarily by1000 ph , which increases as the frequency of interactions between the atoms and the lattice decreases. In fact, lattice thermal conduction is the dominant thermal conduction mechanism in nonmetals, if not the only i. In solids, atoms vibrate near their equilibrium positions (crystal lattice). The vibrations of atoms are not independent of each other, but are rather strongly coupled with neighboring atoms. The regularity of the lattice organisation has an important effect ong ph , with crystalline (well-ordered) materials likequartz having a higher thermal conductivity than amorphous materials similar drinking glass. At sufficiently high temperatures kph ∝ i/T.
For nonmetallic solids,thou is adamant primarily by1000 ph , which increases as the frequency of interactions between the atoms and the lattice decreases. In fact, lattice thermal conduction is the dominant thermal conduction mechanism in nonmetals, if not the only i. In solids, atoms vibrate near their equilibrium positions (crystal lattice). The vibrations of atoms are not independent of each other, but are rather strongly coupled with neighboring atoms. The regularity of the lattice organisation has an important effect ong ph , with crystalline (well-ordered) materials likequartz having a higher thermal conductivity than amorphous materials similar drinking glass. At sufficiently high temperatures kph ∝ i/T.
Thequanta of the crystal vibrational field are referred to as ''phonons.'' A phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, like solids and some liquids. Phonons play a major part in many of the physical properties of condensed matter, like thermal electrical conductivity and electric conductivity. In fact, for crystalline, nonmetallic solids such as diamond, kph can be quite large, exceeding values of yard associated with skilful conductors, such every bit aluminum. In particular, diamond has the highest hardness and thermal conductivity (k = k West/k.K) of any majority cloth.
Thermal Conductivity of Liquids and Gases
In physics, a fluid is a substance that continually deforms (flows) under an applied shear stress.Fluids are a subset of the phases of thing and includeliquids,gases, plasmas and, to some extent, plastic solids. Because the intermolecular spacing is much larger and the motion of the molecules is more random for the fluid state than for the solid state,thermal energy transport is less effective. Thethermal conductivity of gases and liquids is therefore more often than not smaller than that of solids. In liquids, the thermal conduction is acquired by atomic or molecular diffusion. In gases, the thermal conduction is caused by improvidence of molecules from college energy level to the lower level.
Thermal Conductivity of Gases
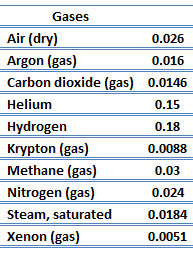 The effect of temperature, pressure, and chemical species on the thermal conductivity of a gas may be explained in terms of thekinetic theory of gases. Air and other gases are generally good insulators, in the absence of convection. Therefore, many insulating materials (due east.m.polystyrene) function simply past having a large number ofgas-filled pockets whichforeclose big-scale convection. Alternation of gas pocket and solid material causes that the heat must be transferred through many interfaces causing rapid decrease in heat transfer coefficient.
The effect of temperature, pressure, and chemical species on the thermal conductivity of a gas may be explained in terms of thekinetic theory of gases. Air and other gases are generally good insulators, in the absence of convection. Therefore, many insulating materials (due east.m.polystyrene) function simply past having a large number ofgas-filled pockets whichforeclose big-scale convection. Alternation of gas pocket and solid material causes that the heat must be transferred through many interfaces causing rapid decrease in heat transfer coefficient.
Thethermal conductivity of gases is directly proportional to the density of the gas, the mean molecular speed, and particularly to themean complimentary path of molecule. The mean free path as well depends on the diameter of the molecule, with larger molecules more likely to experience collisions than small molecules, which is the average distance traveled by an free energy carrier (a molecule) before experiencing a collision. Light gases, such ashydrogen andhelium typically haveloftier thermal conductivity. Dense gases such equally xenon and dichlorodifluoromethane have low thermal conductivity.
In general, the thermal electrical conductivity of gases increases with increasing temperature.
Thermal Conductivity of Liquids
Equally was written, in liquids, the thermal conduction is acquired by atomic or molecular diffusion, merely concrete mechanisms for explaining the thermal conductivity of liquids are not well understood. Liquids tend to have better thermal conductivity than gases, and the ability to catamenia makes a liquid suitable for removing backlog heat from mechanical components. The heat tin can be removed by channeling the liquid through a estrus exchanger. The coolants used in nuclear reactors include water or liquid metals, such as sodium or atomic number 82.
The thermal conductivity of nonmetallic liquids generally decreases with increasing temperature.
Coefficient of Thermal Expansion of Sodium
Linear thermal expansion coefficient of Sodium is 71 µm/(m·One thousand)
Thermal expansion is mostly the trend of matter to change its dimensions in response to a change in temperature. Information technology is ordinarily expressed every bit a fractional modify in length or volume per unit temperature modify. Thermal expansion is common for solids, liquids and for gases. Different gases or liquids, solid materials tend to go on their shape when undergoing thermal expansion. A linear expansion coefficient is unremarkably employed in describing the expansion of a solid, while a book expansion coefficient is more useful for a liquid or a gas.
The linear thermal expansion coefficient is defined as:
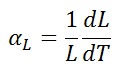
where 50 is a detail length measurement and dL/dT is the rate of alter of that linear dimension per unit alter in temperature.
The volumetric thermal expansion coefficient is the about basic thermal expansion coefficient, and the most relevant for fluids. In general, substances aggrandize or contract when their temperature changes, with expansion or contraction occurring in all directions.
The volumetric thermal expansion coefficient is divers as:
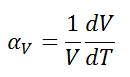
where L is the book of the fabric and dV/dT is the rate of alter of that volume per unit change in temperature.
In a solid or liquid, at that place is a dynamic residue betwixt the cohesive forces holding the atoms or molecules together and the conditions created by temperature. Therefore higher temperatures imply greater distance betwixt atoms. Dissimilar materials take different bonding forces and therefore dissimilar expansion coefficients. If a crystalline solid is isometric (has the aforementioned structural configuration throughout), the expansion will exist uniform in all dimensions of the crystal. For these materials, the area and volumetric thermal expansion coefficient are, respectively, approximately twice and iii times larger than the linear thermal expansion coefficient (αV = 3αL ). If it is not isometric, there may be dissimilar expansion coefficients for different crystallographic directions, and the crystal will change shape as the temperature changes.
Sodium – Specific Oestrus, Latent Estrus of Fusion, Latent Heat of Vaporization
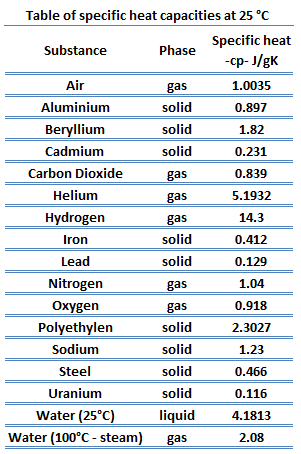
Specific heat of Sodium is ane.23 J/g One thousand.
Latent Heat of Fusion of Sodium is ii.598 kJ/mol.
Latent Oestrus of Vaporization of Sodium is 96.96 kJ/mol.
Specific Rut
Specific heat, or specific heat capacity,is a property related to internal free energy that is very important in thermodynamics. Theintensive propertiescv and cp are defined for pure, elementary compressible substances as partial derivatives of theinternal energyu(T, 5) andenthalpyh(T, p) , respectively:
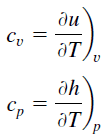
 where the subscriptsv andp denote the variables held fixed during differentiation. The propertiesc5andcp are referred to asspecific heats(orheat capacities) considering under certain special weather they relate the temperature change of a arrangement to the amount of energy added by heat transfer. Their SI units areJ/kg Grand orJ/mol K.
where the subscriptsv andp denote the variables held fixed during differentiation. The propertiesc5andcp are referred to asspecific heats(orheat capacities) considering under certain special weather they relate the temperature change of a arrangement to the amount of energy added by heat transfer. Their SI units areJ/kg Grand orJ/mol K.
Different substancesare affected todifferent magnitudesby theaddition of heat. When a given amount of estrus is added to different substances, their temperatures increase by different amounts.
Estrus capacity is an extensive property of matter, meaning it is proportional to the size of the organization.Rut capacity C has the unit of energy per degree or energy per kelvin. When expressing the same phenomenon as an intensive property, theheat chapters is divided by the corporeality of substance, mass, or volume, thus the quantity is independent of the size or extent of the sample.
Latent Heat of Vaporization
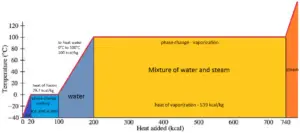 In general, when a materialchanges phase from solid to liquid, or from liquid to gas a certain corporeality of energy is involved in this change of phase. In case of liquid to gas stage change, this amount of energy is known as theenthalpy of vaporization, (symbol ∆Hvap; unit: J) too known every bit the(latent) heat of vaporization or heat of evaporation. As an example, come across the figure, which descibes phase transitions of water.
In general, when a materialchanges phase from solid to liquid, or from liquid to gas a certain corporeality of energy is involved in this change of phase. In case of liquid to gas stage change, this amount of energy is known as theenthalpy of vaporization, (symbol ∆Hvap; unit: J) too known every bit the(latent) heat of vaporization or heat of evaporation. As an example, come across the figure, which descibes phase transitions of water.
Latent rut is the amount of estrus added to or removed from a substance to produce a change in stage. This free energy breaks downwards the intermolecular bonny forces, and likewise must provide the energy necessary to expand the gas (thepΔV work). When latent oestrus is added, no temperature change occurs. The enthalpy of vaporization is a function of the force per unit area at which that transformation takes place.
The temperature at which vaporization (boiling) starts to occur for a given pressure is likewise known as the saturation temperature and at this conditions a mixture of vapor and liquid tin can exist together. The liquid can be said to be saturated with thermal energy. Whatever add-on of thermal energy results in a stage transition. At the boiling point the two phases of a substance, liquid and vapor, have identical free energies and therefore are equally likely to exist. Beneath the humid betoken, the liquid is the more stable state of the two, whereas in a higher place the gaseous form is preferred.
Latent Estrus of Fusion
In case of solid to liquid phase alter, the modify in enthalpy required to change its land is known as the enthalpy of fusion, (symbol ∆Hfus; unit: J) as well known as the(latent) heat of fusion. Latent heat is the corporeality of heat added to or removed from a substance to produce a alter in stage. This energy breaks downwards the intermolecular bonny forces, and also must provide the energy necessary to expand the organisation (thepΔV piece of work).
The liquid phase has a higher internal free energy than the solid stage. This means energy must exist supplied to a solid in social club to melt it and free energy is released from a liquid when it freezes, because the molecules in the liquid experience weaker intermolecular forces and so take a higher potential energy (a kind of bond-dissociation free energy for intermolecular forces).
The temperature at which the phase transition occurs is the melting point. The melting point also defines a condition in which the solid and liquid can be in equilibrium. Calculation a heat will catechumen the solid into a liquid with no temperature change. At the melting point the ii phases of a substance, liquid and vapor, take identical complimentary energies and therefore are equally likely to exist. Below the melting point, the solid is the more stable land of the ii, whereas above the liquid grade is preferred. The melting point of a substance depends on pressure and is commonly specified at standard force per unit area. When considered every bit the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization bespeak.
Sodium – Electrical Resistivity and Electric Conductivity
Electrical resistivity of Sodium is 47.7 nΩ·yard.
Electrical resistivity and its converse, electrical conductivity, is a fundamental belongings of a fabric that quantifies how strongly it resists or conducts the flow of electric current. A depression resistivity indicates a material that readily allows the period of electric current. The symbol of resistivity is usually the Greek letter ρ (rho). The SI unit of electrical resistivity is the ohm-metre (Ω⋅yard). Note that, electrical resistivity is non the same as electrical resistance. Electric resistance is expressed in Ohms. While resistivity is a textile property, resistance is the property of an object.
Conductors – Semiconductors – Resistors
Substances in which electricity can menses are called conductors. Conductors are fabricated of high-conductivity materials such equally metals, in item copper and aluminium.
Insulators, on the other hand, are made of a broad diversity of materials depending on factors such as the desired resistance.
Semiconductors are materials, inorganic or organic, which have the ability to control their conduction depending on chemical structure, temperature, illumination, and presence of dopants. The proper noun semiconductor comes from the fact that these materials take an electrical conductivity between that of a metal, like copper, gold, etc. and an insulator, such equally drinking glass. They have an energy gap less than 4eV (about 1eV). In solid-country physics, this energy gap or band gap is an energy range between valence ring and conduction ring where electron states are forbidden. In dissimilarity to conductors, electrons in a semiconductor must obtain free energy (e.chiliad. from ionizing radiation) to cross the band gap and to reach the conduction band.
To sympathise the difference betwixtmetals,semiconductors andelectrical insulators, we take to define the following terms from solid-state physics:
-
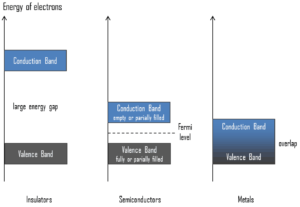 Valence Band. In solid-state physics, thevalence band andconduction band are the bands closest to theFermi level and thus determine the electrical conductivity of the solid. In electrical insulators and semiconductors, the valence band is the highest range of electron energies in which electrons are commonly nowadays at absolute nothing temperature. For example, asilicon atom has fourteen electrons. In the footing country, they are arranged in the electron configuration[Ne]3s23p2 . Of these,iv are valence electrons, occupying the 3s orbital and two of the 3p orbitals. The distinction betwixt the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that have on the properties of both the valence and conduction bands.
Valence Band. In solid-state physics, thevalence band andconduction band are the bands closest to theFermi level and thus determine the electrical conductivity of the solid. In electrical insulators and semiconductors, the valence band is the highest range of electron energies in which electrons are commonly nowadays at absolute nothing temperature. For example, asilicon atom has fourteen electrons. In the footing country, they are arranged in the electron configuration[Ne]3s23p2 . Of these,iv are valence electrons, occupying the 3s orbital and two of the 3p orbitals. The distinction betwixt the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that have on the properties of both the valence and conduction bands. - Conduction Band. In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level and thus determine the electric electrical conductivity of the solid. In electric insulators and semiconductors, theconduction band is the lowest range ofvacant electronic states. On a graph of the electronic band construction of a material, the valence ring is located below the Fermi level, while theconduction ring is located above it. In semiconductors, electrons may achieve the conduction band, when they areexcited, for case, by ionizing radiation (i.e. they must obtain energy higher thanEgap ). For example, diamond is a wide-ring gap semiconductor (Egap= 5.47 eV) with high potential as an electronic device material in many devices. On the other side, germanium has a small band gap energy (Due eastgap = 0.67 eV), which requires to operate the detector at cryogenic temperatures. The distinction betwixt the valence and conduction bands is meaningless in metals, because conduction occurs in one or more than partially filled bands that take on the backdrop of both the valence and conduction bands.
- Band Gap. In solid-land physics, theenergy gap or theband gap is an free energy range between valence band and conduction band where electron states are forbidden. In contrast to conductors, electrons in a semiconductor must obtain energy (due east.1000. from ionizing radiation) to cross the band gap and to attain the conduction band.Band gaps are naturally dissimilar for dissimilar materials. For example, diamond is a broad-band gap semiconductor (Eastgap= 5.47 eV) with high potential as an electronic device material in many devices. On the other side, germanium has a small-scale band gap energy (Egap = 0.67 eV), which requires to operate the detector at cryogenic temperatures.
- Fermi Level. The term "Fermi level" comes fromFermi-Dirac statistics, which describes a distribution of particles over energy states in systems consisting of fermions (electrons) that obey the Pauli exclusion principle. Since they cannot be in identical energy states, Fermi level is the term used to describe the top of the collection ofelectron energy levels at accented cipher temperature. TheFermi level is the surface ofFermi body of water at absolute zero where no electrons volition take plenty energy to rise higher up the surface. In metals, the Fermi level lies in the hypothetical conduction band giving ascent to costless conduction electrons. In semiconductors the position of the Fermi level is within the band gap, approximately in the centre of the band gap.
-
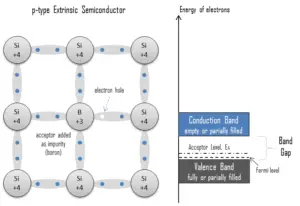 Electron-hole Pair. In the semiconductor,gratis charge carriers areelectrons andelectron holes(electron-hole pairs). Electrons and holes are created byexcitation of electron from valence ring to the conduction band. An electron pigsty (often simply called a hole) is the lack of an electron at a position where one could exist in an atom or diminutive lattice. Information technology is 1 of the two types of charge carriers that are responsible for creating electrical electric current in semiconducting materials. Since in a normal cantlet or crystal lattice the negative accuse of the electrons is balanced past the positive charge of the atomic nuclei, the absence of an electron leaves a net positive charge at the hole'south location. Positively charged holes can movement from atom to cantlet in semiconducting materials as electrons get out their positions. When an electron meets with a hole, they recombine and these free carriers effectively vanish. The recombination means an electron which has been excited from the valence ring to the conduction ring falls back to the empty state in the valence band, known as the holes.
Electron-hole Pair. In the semiconductor,gratis charge carriers areelectrons andelectron holes(electron-hole pairs). Electrons and holes are created byexcitation of electron from valence ring to the conduction band. An electron pigsty (often simply called a hole) is the lack of an electron at a position where one could exist in an atom or diminutive lattice. Information technology is 1 of the two types of charge carriers that are responsible for creating electrical electric current in semiconducting materials. Since in a normal cantlet or crystal lattice the negative accuse of the electrons is balanced past the positive charge of the atomic nuclei, the absence of an electron leaves a net positive charge at the hole'south location. Positively charged holes can movement from atom to cantlet in semiconducting materials as electrons get out their positions. When an electron meets with a hole, they recombine and these free carriers effectively vanish. The recombination means an electron which has been excited from the valence ring to the conduction ring falls back to the empty state in the valence band, known as the holes.
Sodium – Crystal Construction
A possible crystal structure of Sodium is body-centered cubic structure.
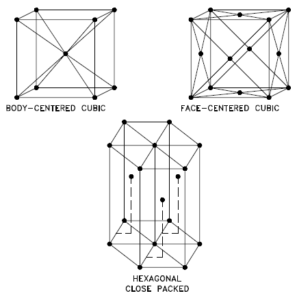
In metals, and in many other solids, the atoms are bundled in regular arrays called crystals. A crystal lattice is a repeating pattern of mathematical points that extends throughout space. The forces of chemical bonding causes this repetition. It is this repeated pattern which control properties like force, ductility, density, electrical conductivity (holding of conducting or transmitting oestrus, electricity, etc.), and shape. There are fourteen general types of such patterns known as Bravais lattices.
The three most mutual bones crystal patterns are:
- Body-centered Cubic. In a body-centered cubic (BCC) arrangement of atoms, the unit cell consists of viii atoms at the corners of a cube and ane atom at the body center of the cube. In a torso-centered cubic organization, a unit of measurement prison cell contains (8 corner atoms × ⅛) + (one center atom × ane) = 2 atoms. The packing is more efficient (68%) than simple cubic and the construction is a common 1 for alkali metals and early on transition metals. Metals containing BCC structures include ferrite, chromium, vanadium, molybdenum, and tungsten. These metals possess high strength and low ductility.
- Face-centered Cubic.In a confront-centered cubic (FCC) arrangement of atoms, the unit cell consists of eight atoms at the corners of a cube and one atom at the center of each of the faces of the cube. In a face-centered cubic arrangement, a unit of measurement prison cell contains (viii corner atoms × ⅛) + (6 face atoms × ½) = iv atoms. This structure, along with its hexagonal relative (hcp), has the virtually efficient packing (74%). Metals containing FCC structures include austenite, aluminum, copper, lead, silver, gold, nickel, platinum, and thorium. These metals possess low strength and loftier ductility.
- Hexagonal Close-packed. In a hexagonal close-packed (HCP) arrangement of atoms, the unit prison cell consists of three layers of atoms. The superlative and lesser layers contain six atoms at the corners of a hexagon and one cantlet at the center of each hexagon. The centre layer contains three atoms nestled between the atoms of the meridian and bottom layers, hence, the name shut-packed. Hexagonal close packed (hcp) is one of the two uncomplicated types of diminutive packing with the highest density, the other beingness the face up centered cubic (fcc). However, unlike the fcc, it is not a Bravais lattice every bit there are two nonequivalent sets of lattice points. Metals containing HCP structures include beryllium, magnesium, zinc, cadmium, cobalt, thallium, and zirconium. HCP metals are non as ductile as FCC metals.
–
How Many Protons In Na,
Source: https://www.periodic-table.org/sodium-periodic-table/
Posted by: ashburnoicieffive97.blogspot.com


0 Response to "How Many Protons In Na"
Post a Comment