Potassium Nitrate Soluble Or Insoluble

Salts

Soluble & Insoluble Salts • • All sodium, potassium and ammonium compounds are soluble. All nitrates are soluble All common ethanoates (also called acetates) are soluble Most common chlorides are soluble, except lead(Two) chloride and silver chloride Most common sulphates are soluble, except lead(II) sulphate, barium sulphate and calcium sulphate Near common carbonates are insoluble, except sodium, potassium and ammonium carbonates Almost metal hydroxides are insoluble, except sodium, potassium and ammonium hydroxides

Preparation of Insoluble Salts The full general method for preparing insoluble salts: i. Choose the two solutions, i containing the cations required to make the salt and the other containing the anions. 2. Mix the two solutions to grade the insoluble table salt as a precipitate. 3. Filter the mixture and collect the precipitate as the residue. iv. Wash the residue with distilled water and leave to dry.

Case - Preparation of an Insoluble Salt Ba. Cl 2(aq) + Na ii So four(aq) → Ba. And then four(due south) + 2 Na. Cl(aq) Ionic Equation: Ba 2+(aq) + SO 42 -(aq) → Ba. SO 4(s)

Preparation of Soluble Salts There are three main methods of preparing soluble salts: 1. Straight combination. 2. The reaction of an acrid with a metal, an insoluble base or an insoluble carbonate. 3. The reaction between an acid and a soluble base (alkali).
(a) Directly Combination Salts composed of two unproblematic ions such as metal chlorides can be prepared by reacting two elements, a metallic and a non-metal, directly with each other. The metal supplies the cations and the non-metal supplies the anions. Example: 2 Al(s) + iii Cl 2(g) → 2 Al. Cl 3(south)

(b) Reaction with an acrid Soluble salts can be prepared by the reaction between and acrid and: • a reactive metal Mg(s) + 2 HCl(aq) → Mg. Cl two(aq) + H 2(g) • an insoluble carbonate Ca. CO 3(south) + 2 HCl(aq) → Ca. Cl 2(aq) + H 2 O(l) + CO 2(g) • an insoluble base Cu. O(south) + H 2 So iv(aq) → Cu. SO 4(aq) + H ii O(l) In these reactions, the final product volition only be a pure solution of the required common salt in water if the reaction has reached completion and no acrid remains.

(c) Reaction between an acid and a soluble alkali Potassium, sodium and ammonium salts are prepared past titrating an acid with an aqueous alkali. In this preparation method, the acid is added to the alkali and the reaction reaches completion when the solution is only neutral. The color change of an indicator is used to determine the neutralisation point.

Some Types of Chemical Reactions

Can you spot three (iii) differences?
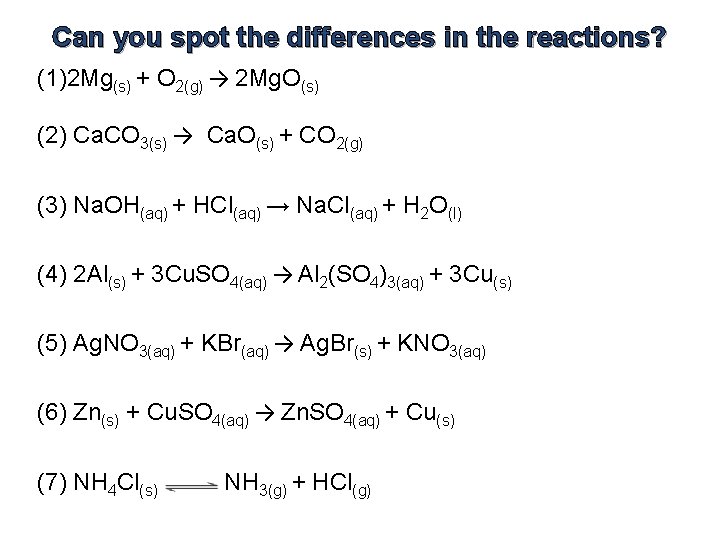
Tin can you spot the differences in the reactions? (1)2 Mg(s) + O 2(g) → 2 Mg. O(south) (2) Ca. CO iii(s) → Ca. O(s) + CO 2(1000) (3) Na. OH(aq) + HCl(aq) → Na. Cl(aq) + H 2 O(l) (4) two Al(s) + 3 Cu. So 4(aq) → Al 2(SO 4)3(aq) + iii Cu(s) (v) Ag. NO 3(aq) + KBr(aq) → Ag. Br(s) + KNO 3(aq) (6) Zn(due south) + Cu. SO 4(aq) → Zn. SO 4(aq) + Cu(s) (7) NH 4 Cl(s) NH 3(g) + HCl(yard)
1. Combination Reactions
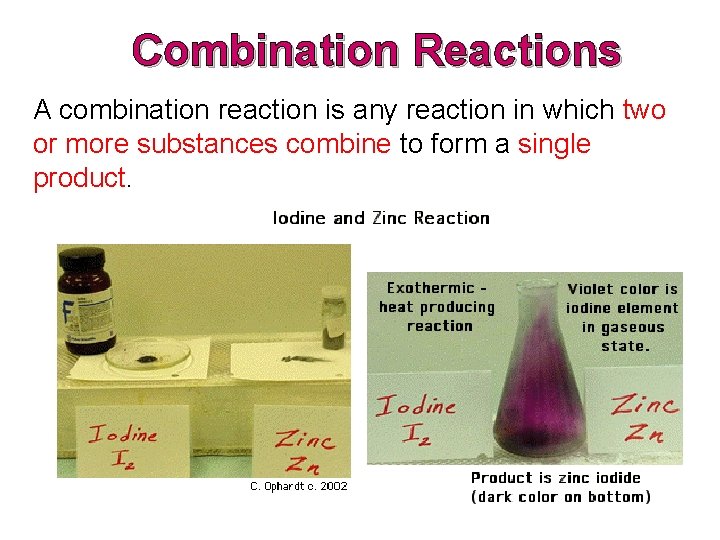
Combination Reactions A combination reaction is whatsoever reaction in which 2 or more substances combine to form a single product.

Examples of Combination Reactions Example 1: magnesium + oxygen → magnesium oxide 2 Mg(s) + O 2(g) → 2 Mg. O(s)

Example 2: hydrogen + oxygen → water two H 2(thousand) + O 2(thousand) → 2 H 2 O(g)
2. Decomposition Reactions

Decomposition Reactions A decomposition reaction is whatever reaction where a single reactant is broken downwards into two or more products. A decomposition reaction will have identify if the compound is unstable, if the compound is heated or if an electrical current is passed through it.

Examples of Decomposition Reactions A decomposition reaction is generally symbolised by: AB → A + B Example 1: calcium carbonate calcium oxide + carbon dioxide Ca. CO 3(south) Ca. O(s) + CO 2(k) Example 2: potassium chlorate due east potassium chloride + oxygen 2 KCl. O iii(south) 2 KCl(southward) + 3 O 2(1000)

iii. Neutralisation Reactions

Neutralisation Reactions Neutralization reactions are reactions between a base of operations (or an brine) and an acid. The acid is neutralized by the base of operations (or alkali) and the products formed are a common salt and h2o.
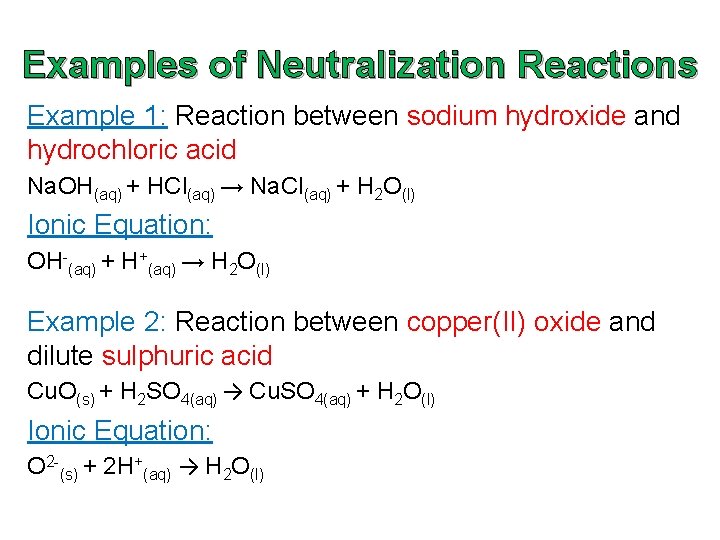
Examples of Neutralization Reactions Example 1: Reaction between sodium hydroxide and hydrochloric acid Na. OH(aq) + HCl(aq) → Na. Cl(aq) + H 2 O(50) Ionic Equation: OH-(aq) + H+(aq) → H 2 O(l) Instance two: Reaction between copper(II) oxide and dilute sulphuric acrid Cu. O(s) + H 2 SO 4(aq) → Cu. And so iv(aq) + H 2 O(50) Ionic Equation: O 2 -(s) + 2 H+(aq) → H 2 O(l)
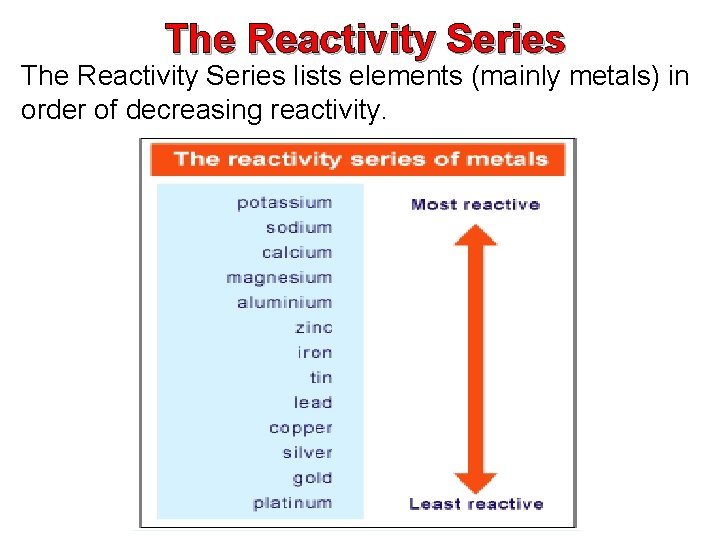
The Reactivity Series lists elements (mainly metals) in order of decreasing reactivity.

4. Unmarried Deportation Reactions
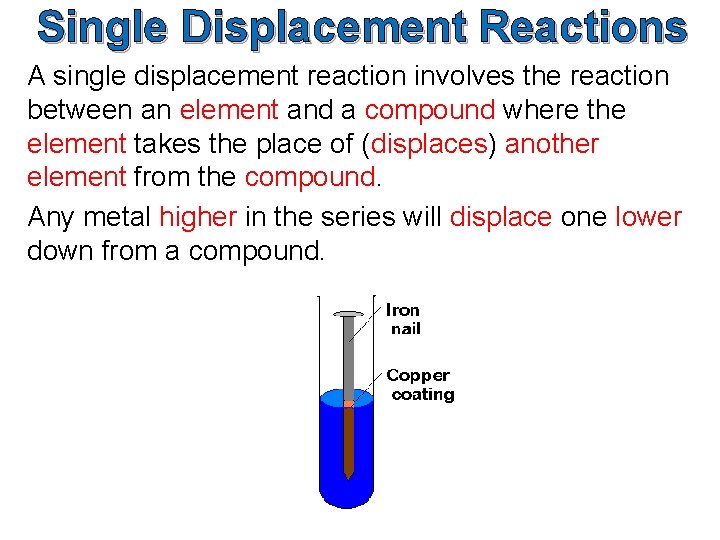
Unmarried Displacement Reactions A unmarried displacement reaction involves the reaction betwixt an element and a compound where the element takes the place of (displaces) another chemical element from the compound. Any metal college in the series will readapt one lower downwardly from a compound.
Examples of Unmarried Deportation Reactions A single deportation reaction is mostly symbolised by: A + BC → Air-conditioning + B Case ane aluminium + copper sulphate → aluminium sulphate + copper 2 Al(s) + iii Cu. So four(aq) → Al 2(So iv)3(aq) + 3 Cu(s) Ionic Equation: 2 Al(due south) + 3 Cu ii+(aq) → Al 3+(aq) + iii Cu(due south) Example two Carbon + copper(II) oxide → carbon dioxide + copper C(southward) + 2 Cu. O(due south) → CO 2(g) + two Cu(s) Ionic Equation: C(southward) + Cu two+(aq) → C 2+(aq) + Cu(s)

Example 3 Magnesium + zinc oxide → magnesium oxide + zinc Mg(s) + Zn. O(southward) → Mg. O(s) + Zn(s) Ionic Equation: Mg(s) + Zn 2+(aq) → Mg two+(aq) + Zn(south)

Example iv Copper + silvery nitrate → copper(II) nitrate + argent Cu(due south) + two Ag. NO 3(aq) → Cu(NO 3)2(aq) + two Ag(s) Ionic Equation: Cu(southward) + ii Ag+(aq) → Cu 2+(aq) + Ag(s)
five. Double Deportation / Ionic Precipitation Reactions

Ionic Atmospheric precipitation Reactions Ionic precipitation reactions are sometimes referred to as double deportation reactions. They generally involve compounds which are in solution, where the compounds substitution ions.

An ionic precipitation reaction is more often than not symbolised past: AB + CD → AD + CB To have a successful ionic precipitation reaction, one or both of the products must be incapable of undergoing the reverse reaction, generally because they form an insoluble precipitate.

Examples of Ionic Precipitation Reactions Example 1: Ag. NO iii(aq) + KBr(aq) → Ag. Br(south) + KNO 3(aq) Ionic Equation: Ag+(aq) + Br-(aq) → Ag. Br(s) Example two: Ba. Cl 2(aq) + Na 2 Then 4(aq) → Ba. SO 4(south) + two Na. Cl(aq) Ionic Equation: Ba 2+(aq) + SO 42 -(aq) → Ba. And so four(south)

six. Redox Reactions

Redox Reactions Redox reactions or oxidation-reduction reactions are chemical reactions in which one reactant is oxidised and the other is reduced or both change is oxidation number.

Case of a Redox Reaction One of many examples is when zinc metal is placed in a solution of copper sulphate, the copper is reduced and appears equally a black coating on the zinc.
7. Reversible Chemical Reactions
7. Reversible Chemical Reactions There are sure chemical reactions which, under certain conditions, are reversible, i. e. the products can react to produce the original reactants again. These are known as reversible chemical reactions. Almost reactions are not reversible; they can but go along in ane management. If a reaction is reversible a double pointer ( )is used. In a reversible reaction we refer to the forward reaction as proceeding from left to right and the reverse reaction as proceeding from right to left.
Example of a Reversible Chemical Reaction When ammonium chloride solid is heated it sublimes into ammonia and hydrogen chloride gas. On cooling the ii gases recombine to from ammonium chloride again. NH 4 Cl(southward) NH 3(g) + HCl(g)

Making Predictions using the Reactivity Series You can brand predictions about unfamiliar metals if you know their position in the reactivity series.

A problem involving manganese Manganese, Mn, lies between aluminium and zinc in the Reactivity Series and forms a 2+ ion. Solutions of manganese(II) salts are very, very pale pink (most colourless). (a) Use the Reactivity Series to predict whether manganese will react with copper(2) sulphate solution. If it will react, draw what you would come across, name the products and write an equation for the reaction. (b) Explain why you would expect manganese to react with steam. Name the products of the reaction and write the equation.

Respond (a)Manganese is above copper in the Reactivity Series then will displace it from the copper(2) sulphate. A dark-brown deposit of copper will be formed. The colour of the solution volition fade from bluish and leave a very pale pink (well-nigh colourless) solution of manganese(II) sulphate. Mn(south) + Cu. SO iv(aq) → Mn. And so 4(aq) + Cu(s)
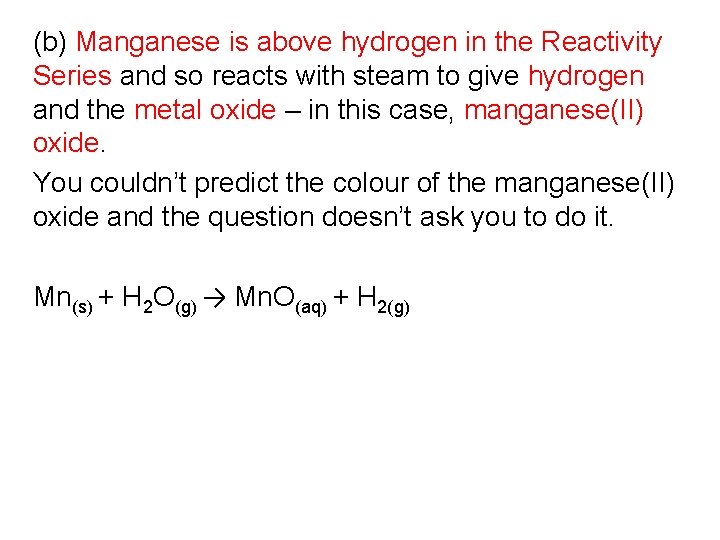
(b) Manganese is above hydrogen in the Reactivity Series and so reacts with steam to give hydrogen and the metallic oxide – in this case, manganese(II) oxide. You couldn't predict the color of the manganese(2) oxide and the question doesn't ask y'all to practise it. Mn(south) + H 2 O(thou) → Mn. O(aq) + H ii(g)
Potassium Nitrate Soluble Or Insoluble,
Source: https://slidetodoc.com/salts-soluble-insoluble-salts-all-sodium-potassium-and/
Posted by: ashburnoicieffive97.blogspot.com


0 Response to "Potassium Nitrate Soluble Or Insoluble"
Post a Comment